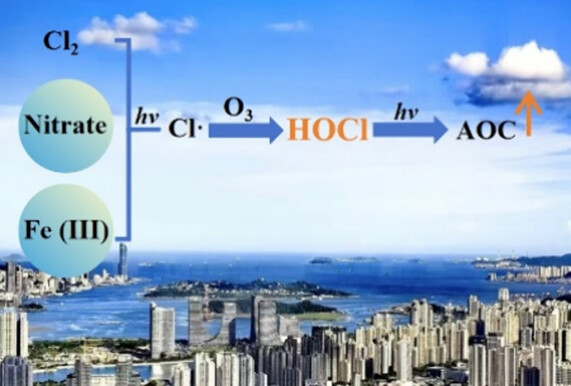
Chlorine (Cl) radicals can profoundly affect the atmospheric oxidation capacity and the fates of pollutants. Hypochlorous acid (HOCl) is a potentially crucial Cl precursor, yet the understanding of its formation mechanisms and atmospheric impacts is still limited. Here, we observed high concentrations of HOCl in a coastal city of Southeast China during the autumn of 2022, with an average daytime peak of 181 ppt. Machine learning analysis identified Cl2, O3, nitrate, temperature, and iron as the primary factors affecting HOCl distribution. Beyond Cl2 photolysis, both nitrate photolysis and aerosol iron photochemistry also contributed to Cl radical production, which drove daytime HOCl production through reactions involving ClO and HO2 radicals in the presence of O3. Both OH and Cl radicals released via HOCl photolysis increased the levels of ROx radicals by ∼10%, thereby enhancing the daytime O3 generation and atmospheric oxidation capacity. Our findings emphasize the significant role of HOCl in atmospheric chemistry and suggest that controlling O3 levels could alleviate Cl radical production and its adverse impacts on air quality.