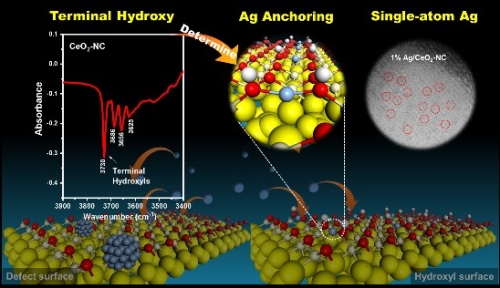
Monoatomic dispersion of precious metals on the surface of CeO2 nanocrystals is a highly practical approach for dramatically reducing the usage of precious metals while exploiting the unique properties of single-atom catalysts. However, the specific atomic sites for anchoring precious metal atoms on the CeO2 support and underlying chemical mechanism remain partially unknown. Herein, we show that the terminal hydroxyls on the (100) surface are the most stable sites for anchoring Ag atoms on CeO2, indicating that CeO2 nanocubes are the most efficient substrates to achieve monoatomic dispersion of Ag. Importantly, the newly identified chemical mechanism for single-metal-atom dispersion on CeO2 nanocubes appears to be generic and can thus be extended to other precious metals (Pt and Pd). In fact, our experiments also show that atomically dispersed Pt/Pd species exhibit morphology- and temperature-dependent CO selectivity in the catalytic CO2 hydrogenation reaction.