

Strong metal-support interactions (SMSI) have gained great attention in the heterogeneous catalysis field, but its negative role in regulating light-induced electron transfer is rarely explored. Herein, we describe how SMSI significantly restrains the activity of Ru/TiO2 in light-driven CO2 reduction by CH4 due to the photo-induced transfer of electrons from TiO2 to Ru. In contrast, on suppression of SMSI Ru/TiO2-H2 achieves a 46-fold CO2 conversion rate compared to Ru/TiO2. For Ru/TiO2-H2, a considerable number of photo-excited hot electrons from Ru nanoparticles (NPs) migrate to oxygen vacancies (OVs) and facilitate CO2 activation under illumination, simultaneously rendering Ruδ+ electron deficient and better able to accelerate CH4 decomposition. Consequently, photothermal catalysis over Ru/TiO2-H2 lowers the activation energy and overcomes the limitations of a purely thermal system. This work offers a novel strategy for designing efficient photothermal catalysts by regulating two-phase interactions.
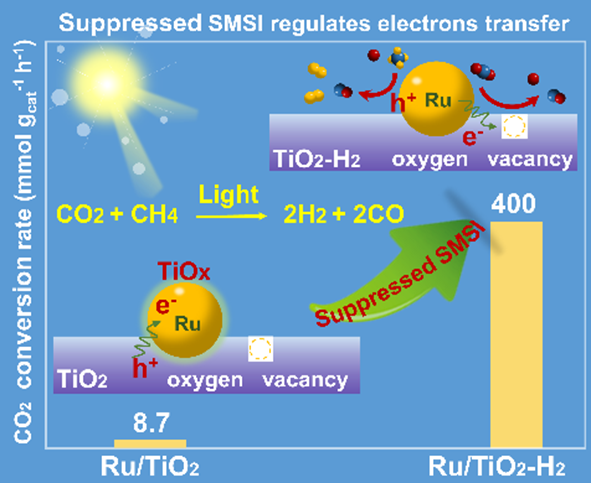
The effect of suppressive SMSI on regulating electrons transfer and the catalytic performance