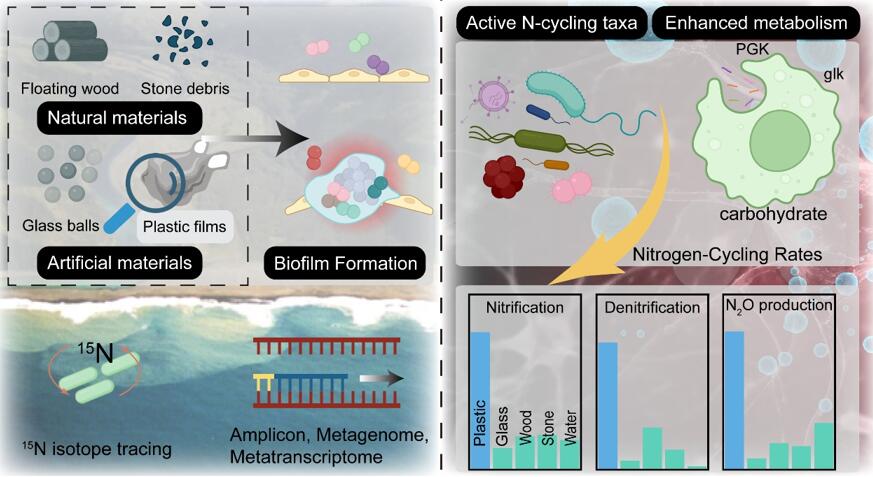
Biofilms represent a ubiquitous microbial lifestyle that facilitates colonization, symbiosis, and nutrient cycling, shaping environmental chemical transformations. In the Anthropocene, the proliferation of artificial surfaces, particularly plastics, has introduced novel and artificial ecological niches for microbial colonization. However, the biogeochemical potential of biofilms on these emerging artificial substrates remains largely unknown. Here, using 15N tracing, amplicon, metagenome, and metatranscriptomic sequencing, we explore nitrogen (N) potential biogeochemistry across artificial and natural biofilms as well as the bulk seawater. Our results reveal that plastic biofilms exhibit enhanced N transformation potential, including elevated nitrification (2 ~ 45-fold), denitrification (5 ~ 44-fold), and N2O production (3 ~ 13-fold) rates, compared to natural biofilms and ambient seawater. This functional shift corresponds to distinct microbial community structures, driven by active N-cycling taxa and metabolic pathway reconfigurations on plastic surfaces. We also observe that carbohydrate metabolism pathways, such as glycolysis and the pentose phosphate pathway, were highly expressed in plastic biofilms, with transcriptional levels of glk (encoding glucokinase) and PGK (encoding phosphoglycerate kinase) increased by 6 and 2-fold, respectively. Our findings depict the role of plastic biofilms as active participants in estuarine N cycling and underscore the broader implications of plastic pollution on ecosystem biogeochemistry.