Global warming is redrawing the map for invasive species, spotlighting the globally harmful giant African snail as a major ecological disruptor and public health threat. Known for harboring extensive antibiotic resistance genes (ARGs) and human pathogens, it remains uncertain whether global warming exacerbates these associated health risks.
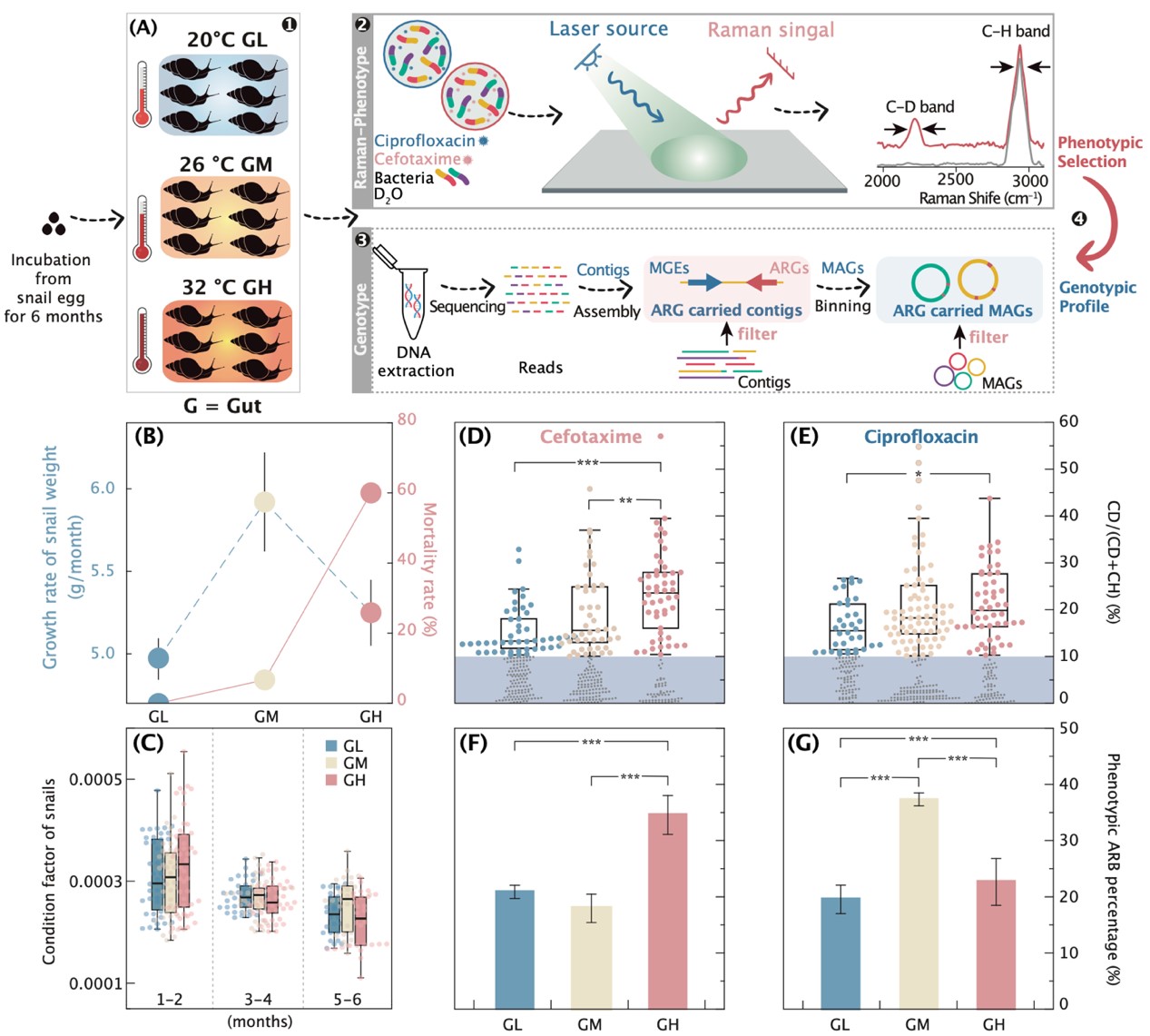
We use phenotype-based single-cell Raman with D2O labeling (Raman-D2O) and genotype-based metagenomic sequencing to investigate whether soil warming increases active antibiotic-resistant bacteria (ARBs) in the gut microbiome of giant African snails.
We show a significant increase in beta-lactam phenotypic resistance of active ARBs with rising soil temperatures, mirrored by a surge in beta-lactamase genes such as SHV, TEM, OCH, OKP, and LEN subtypes. Through a correlation analysis between the abundance of phenotypically active ARBs and genotypically ARG-carrying gut microbes, we identify species that contribute to the increased activity of antibiotic resistome under soil warming. Among 299 high-quality ARG-carrying metagenome-assembled genomes (MAGs), we further revealed that the soil warming enhances the abundance of “supercarriers” including human pathogens with multiple ARGs and virulence factors. Furthermore, we identified elevated biosynthetic gene clusters (BGCs) within these ARG-carrying MAGs, with a third encoding at least one BGC. This suggests a link between active ARBs and secondary metabolism, enhancing the environmental adaptability and competitive advantage of these organisms in warmer environments.
The study underscores the complex interactions between soil warming and antibiotic resistance in the gut microbiome of the giant African snail, highlighting a potential escalation in environmental health risks due to global warming. These findings emphasize the urgent need for integrated environmental and health strategies to manage the rising threat of antibiotic resistance in the context of global climate change.